Mortality Not Correlated With Paclitaxel Exposure: An Independent Patient-Level Meta-Analysis of a Drug-Coated Balloon
Background
Five years of prospective clinical trials confirm that the paclitaxel drug-coated balloon (DCB) (IN.PACT Admiral, Medtronic, Dublin, Ireland) is safe and effective to treat femoropopliteal artery disease. A recent meta-analysis of heterogeneous trials of paclitaxel-based balloons and stents reported that they are associated with increased mortality and that higher doses are linked to higher mortality from 2 to 5 years.
Objectives
The purpose of this study was to determine if there is a correlation between paclitaxel exposure and mortality by conducting an independent patient-level meta-analysis of 1,980 patients with up to 5-year follow-up.
Methods
Data from 2 single-arm and 2 randomized independently adjudicated prospective studies of a paclitaxel DCB (n = 1,837) and uncoated percutaneous transluminal angioplasty (PTA) (n = 143) were included. Analyses of baseline, procedure, and follow-up data of individual patients were performed to explore correlations of paclitaxel dose with long-term mortality. Survival time by paclitaxel dose tercile was analyzed with adjustment of inverse probability weighting to correct baseline imbalances and study as random effect. A standard cohort was defined to compare DCB- and PTA-treated patients with similar characteristics by applying criteria from pivotal studies (n = 712 DCB, n = 143 PTA).
Results
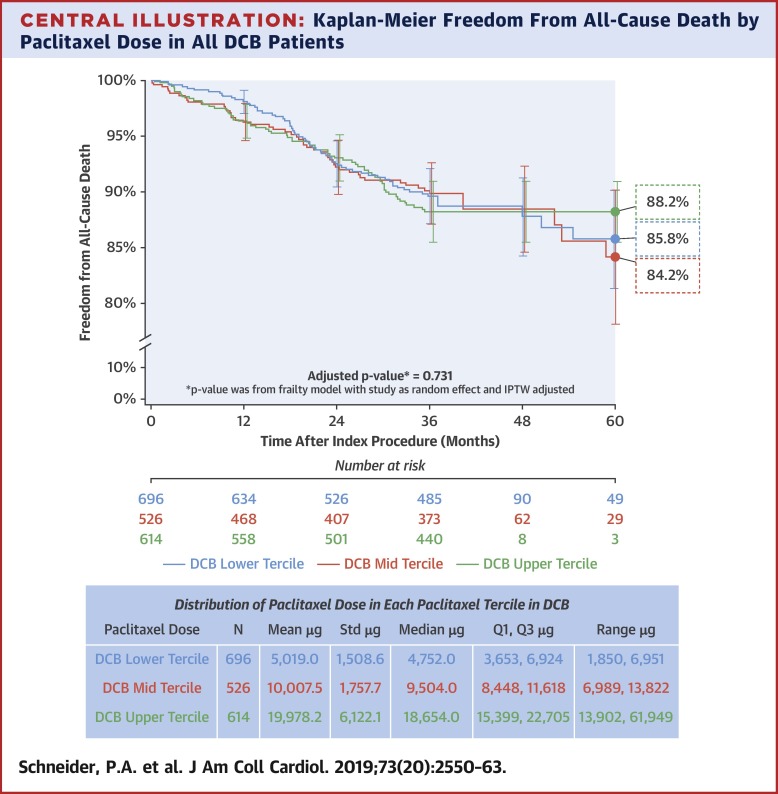
A survival analysis stratified nominal paclitaxel dose by low, mid, and upper terciles; mean doses were 5,019.0, 10,007.5, and 19,978.2 μg, respectively. Rates of freedom from all-cause mortality between the 3 groups through 5 years were 85.8%, 84.2%, and 88.2%, respectively (p = 0.731). There was no significant difference in all-cause mortality between DCB and PTA through 5 years comparing all patients (unadjusted p = 0.092) or patients with similar characteristics (adjusted p = 0.188).
Conclusions
This independent patient-level meta-analysis demonstrates that this paclitaxel DCB is safe. Within DCB patients, there was no correlation between level of paclitaxel exposure and mortality. (Randomized Trial of IN.PACT Admiral® Drug Coated Balloon vs Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease [INPACT SFA I], NCT01175850; IN.PACT Admiral Drug-Coated Balloon vs. Standard Balloon Angioplasty for the Treatment of Superficial Femoral Artery [SFA] and Proximal Popliteal Artery [PPA] [INPACT SFA II], NCT01566461; MDT-2113 Drug-Eluting Balloon vs. Standard PTA for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery and/or Proximal Popliteal Artery [MDT-2113 SFA], NCT01947478; The IN.PACT SFA Clinical Study for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery and/or Proximal Popliteal Artery Using the IN.PACT Admiral™ Drug-Eluting Balloon in a Chinese Patient Population, NCT02118532; and IN.PACT Global Clinical Study, NCT01609296)
Central Illustration