






Berlin, 16. April 2020 – Patientinnen und Patienten mit Diabetischem Fußsyndrom können sich vor einer Amputation an den unteren Extremitäten zukünftig eine unabhängige ärztliche Zweitmeinung einholen. Hierbei überprüft ein qualifizierter Zweitmeiner die medizinische Notwendigkeit des geplanten Eingriffs und berät zu konservativen und weniger invasiven Behandlungsmöglichkeiten. Die entsprechende Ergänzung der Richtlinie zum Zweitmeinungsverfahren beschloss der Gemeinsame Bundesausschuss (G-BA) am Donnerstag in Berlin. Zudem beauftragte der G-BA das Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG), wissenschaftlich fundierte und unabhängige Gesundheitsinformationen zum Thema Amputation beim Diabetischen Fußsyndrom zu erstellen und auf seiner Website zu veröffentlichen.
Bei Patientinnen und Patienten mit Diabetes mellitus kann es aufgrund von Schäden an den kleinen Blutgefäßen und Nerven der Füße zu einem Diabetischen Fußsyndrom kommen. Das Diabetische Fußsyndrom ist durch eine schlecht heilende Wunde am Fuß gekennzeichnet, die im höheren Stadium bis auf die Ebene der Knochen reichen kann. Eine Behandlungsstrategie ist die Amputation an der unteren Extremität bis unterhalb der Knöchelregion oder oberhalb der Knöchelregion. Zu den alternativen Vorgehensweisen gehören die chirurgische Reinigung der Wunde, die Druckentlastung, die Behandlung von Infektionen und die Durchblutungsförderung.
Gegenstand des neuen Zweitmeinungsverfahrens ist die ärztliche Empfehlung für eine Amputation an der unteren Extremität zur Behandlung des Diabetischen Fußsyndroms. Es zielt darauf ab, eine informierte Entscheidungsfindung der Patientinnen und Patienten bei der Auswahl weniger invasiver oder konservativen Behandlungsmöglichkeiten zu unterstützen und eine medizinisch nicht gebotene Amputation zu vermeiden.
Die Genehmigung, Zweitmeinungsleistungen zu einer Amputation beim Diabetischen Fußsyndrom abzurechnen, können Fachärztinnen und Fachärzte folgender Fachrichtungen bei ihrer Kassenärztlichen Vereinigung beantragen:
Fachärztinnen und Fachärzten, die aufgrund ihrer besonderen Qualifikation und Unabhängigkeit für den jeweiligen Eingriff eine Genehmigung als Zweitmeinungsgeber erhalten, werden auf der Website des ärztlichen Bereitschaftsdienstes unter www.116117.de/zweitmeinung zu finden sein.
Der Beschluss wird nun dem Bundesministerium für Gesundheit (BMG) zur rechtlichen Prüfung vorgelegt. Er tritt nach Nichtbeanstandung durch das BMG und Veröffentlichung im Bundesanzeiger in Kraft.
TINTIN is a physician initiated, prospective, single arm multicenter, Belgian trial, that investigates the safety and efficacy of the treatment with luminor DCB and iVolution self-expanding stent in TASC C and D femoropopliteal lesions of 240mm average.
The 1-year clinical outcomes of TINTIN Trial were presented at LINC 2020 by Dr Koen Deloose (Dendermonde, Belgium) who stated, during his presentation, that: “The combination of luminor DCB and iVolution self-expanding stent, shows impressive 1-year outcomes in the TINTIN trial, comparable to DEs but in more complex lesions”.
TINTIN 1-year outcomes show a Primary Patency of 90.5%, freedom from TLR of 94.4%. Safety has been proved with 0 device related deaths.
Incidental findings during computed tomographicangiography diagnostic work-up in patients witharteriogenic erectile dysfunction:
» Official Article Swiss Medical Weekly «
Summary
AIM: To analyse the incidental findings during computertomographic angiography (CTA) diagnostic work-up in patients with arteriogenic erectile dysfunction (ED).
PATIENTS AND METHODS: The medical records of all patients with suspected arteriogenic ED were entered into a database. Risk factors and underlying comorbidities were also collected. Pathological CTA findings were extracted from the CT readings and entered into the data-base. Incidental findings on CTA were classified as those requiring immediate medical treatment, requiring deferred medical treatment or of no clinical importance.
RESULTS: A total of 200 patients underwent CTA for suspected arteriogenic ED. Mean patient age was 59.6 ± 11.7 years. Of these, 181 patients (90.5%) had obstructions of erection-related arteries. In 168 patients (84.0%), CTA showed multiple incidental pathological findings. Eighty-five of 200 patients (42.5%) exhibited incidental findings requiring immediate further medical workup and/or treatment: coronary artery calcification was diagnosed in 75/200 (37.5%), aorto-iliac aneurysms in 8/200 (4%) of patients and incidentally detected embolism in 1/200 patient. Pancreatic and liver tumours were less frequent (incidence 1.5% and 1%, respectively). Incidental findings requiring deferred medical workup and/or treatment were detected in 175/200 patients (87.5%). The findings with the highest prevalence were liver steatosis followed bycolon diverticulosis and prostate hyperplasia. Findings of little to no clinical importance were reported in 117 (58.5%) patients. These included uncomplicated renal cysts, spinal degeneration and renal vascular anomalies. Almost every second patient presenting with ED had an incidental find-ing which required immediate treatment.
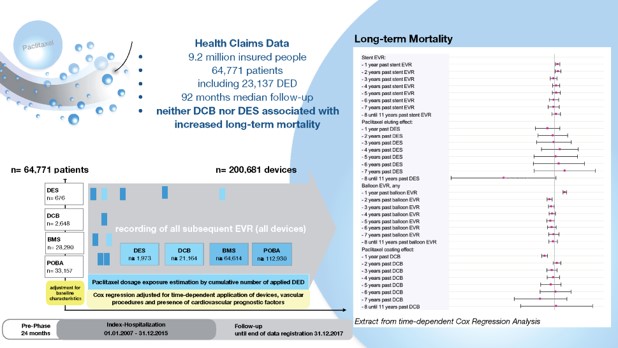
A real-world analysis of 9.2 million patients included in a German insurance database showed no evidence for increased mortality associated with paclitaxel-based drug-eluting devices (DEDs) during 11 years of use in endovascular revascularization (EVR) of peripheral vessels. The findings from the analysis were published by Eva Freisinger, MD, et al in European Heart Journal.
According to the investigators, the study sought to address the safety concerns raised by recently published data that indicated a two-fold increase in long-term mortality in patients treated with these devices. The concerns caused international regulatory authorities to issue several alerts for further application of paclitaxel-based DEDs.
As summarized in European Heart Journal, insurants of Barmer Health Insurance were included in the data on the application of paclitaxel-based drug-eluting stents (DESs) and drug-coated balloons (DCBs) and were retrieved from their introduction on the market in 2007 until present.
All patients who underwent their first EVR between 2007 and 2015 were indexed and followed until December 31, 2017. Each subsequently applied DES, DCB, bare-metal stent, and uncoated balloon was included in further analyses. Multivariable Cox regression analysis considered potential nonlinear time-dependent hazard ratios (HR) of DES and DCB use over 11 years.
The investigators identified 64,771 patients who underwent 107,112 EVR procedures using 23,137 DEDs. Multivariable Cox regression analysis showed that paclitaxel-based DESs were not associated with increased long-term mortality for over 11 years after application (all P > .057). DCB use was associated with decreased long-term mortality for the first year after application (HR, 0.92; P < .001) and indifferent correlation in the years thereafter (all P > .202), reported the investigators in European Heart Journal.
[Endovascular Today, October 8, 2019]
The new data, presented for the first time at the Annual Scientific Meeting of the Cardiovascular and Interventional Radiological Society of Europe (CIRSE 2019; 7–11 September, Barcelona, Spain), also showed a significant clinical and haemodynamic improvement from baseline, similar to the level of improvement achieved with POBA, but with considerably fewer repeat revascularisations. The investigators found no difference to POBA regarding safety.
The EffPac (Effectiveness of paclitaxel-coated Luminor balloon catheter versus uncoated balloon catheter in the arteria femoralis superficialis) trial was designed to assess the safety and efficacy of the Luminor paclitaxel drug-eluting balloon in inhibiting restenosis and in ensuring long-term patency. The investigators compared the DCB catheter to non-coated PTA in stenotic or occlusive lesions of the femoropopoliteal artery.
Between September 2015 and December 2016, the investigators enrolled 171 subjects across 11 German study centres. Eighty-five of these patients were randomised to DCB angioplasty, with the remaining 86 being assigned to the PTA group. They note that before the 24-month mark, analysis was discontinued for 25 patients in the DCB group and 30 patients in the POBA cohort.
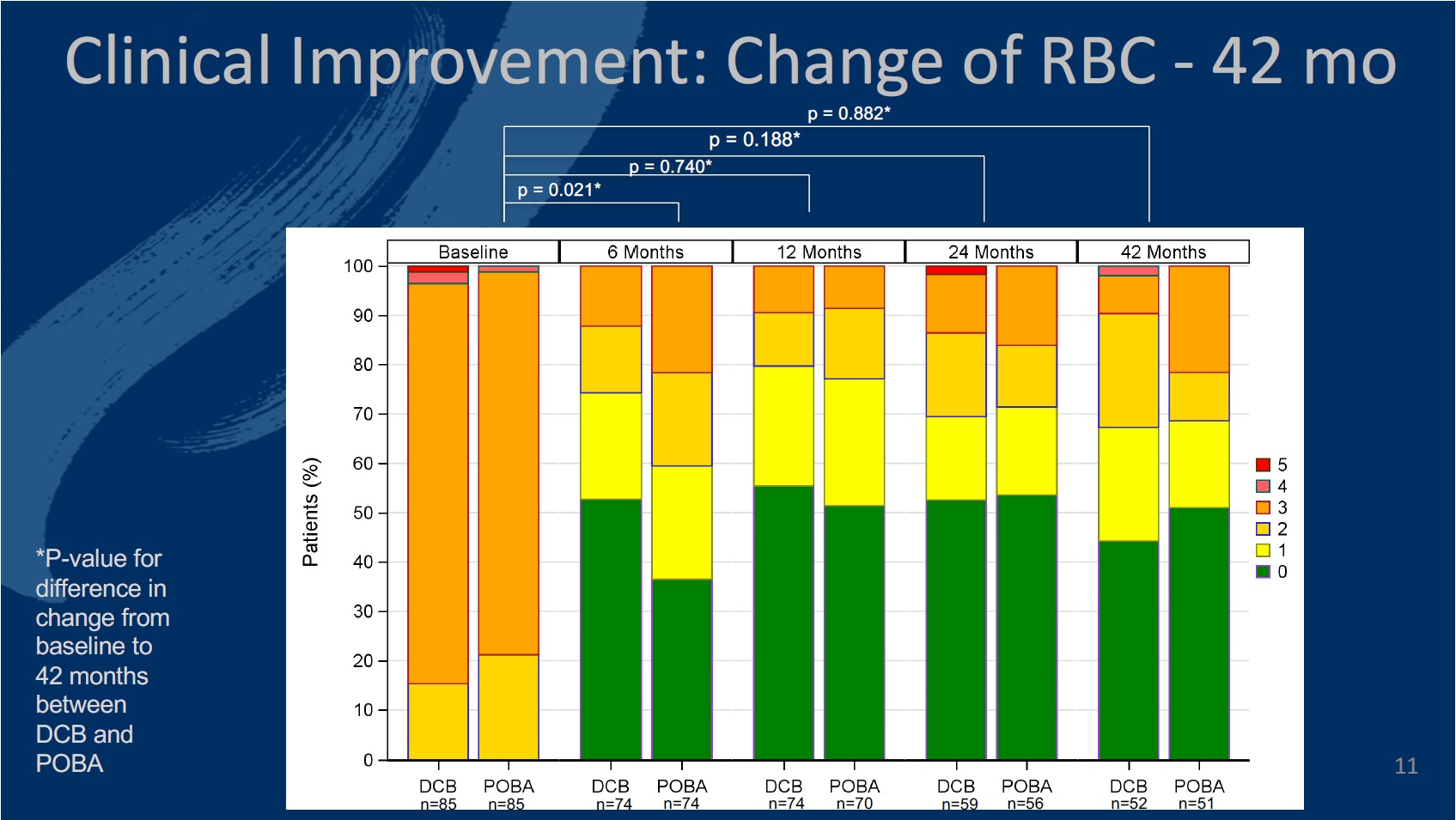
The primary endpoint of the study was defined as late lumen loss at six months. The secondary endpoints at six, 12, and 24 months included primary patency, target lesion (TLR) and vessel (TVR) revascularisation, quality of life—assessed by the EQ-5D—change of Rutherford-Becker classification (RBC), ankle-brachial index (ABI), and major and minor amputation rates, among others.
The authors comment that 12 months in to the study, the investigational DCB catheter proved “highly effective and safe in inhibiting restenosis,” and demonstrated a walking improvement in change of RBC compared to PTA.
Twelve-month results of the safety and efficacy of the Luminor device had been released prior to the CIRSE 2019 presentation. The authors detail that at six months, the late lumen loss was on average 0.92mm lower in the DCB group than in the PTA group (95% confidence interval [CI]: -1.36mm; -0.49mm, p<0.001). Significantly more patients showed a walking improvement after DCB treatment at six months (p=0.021).
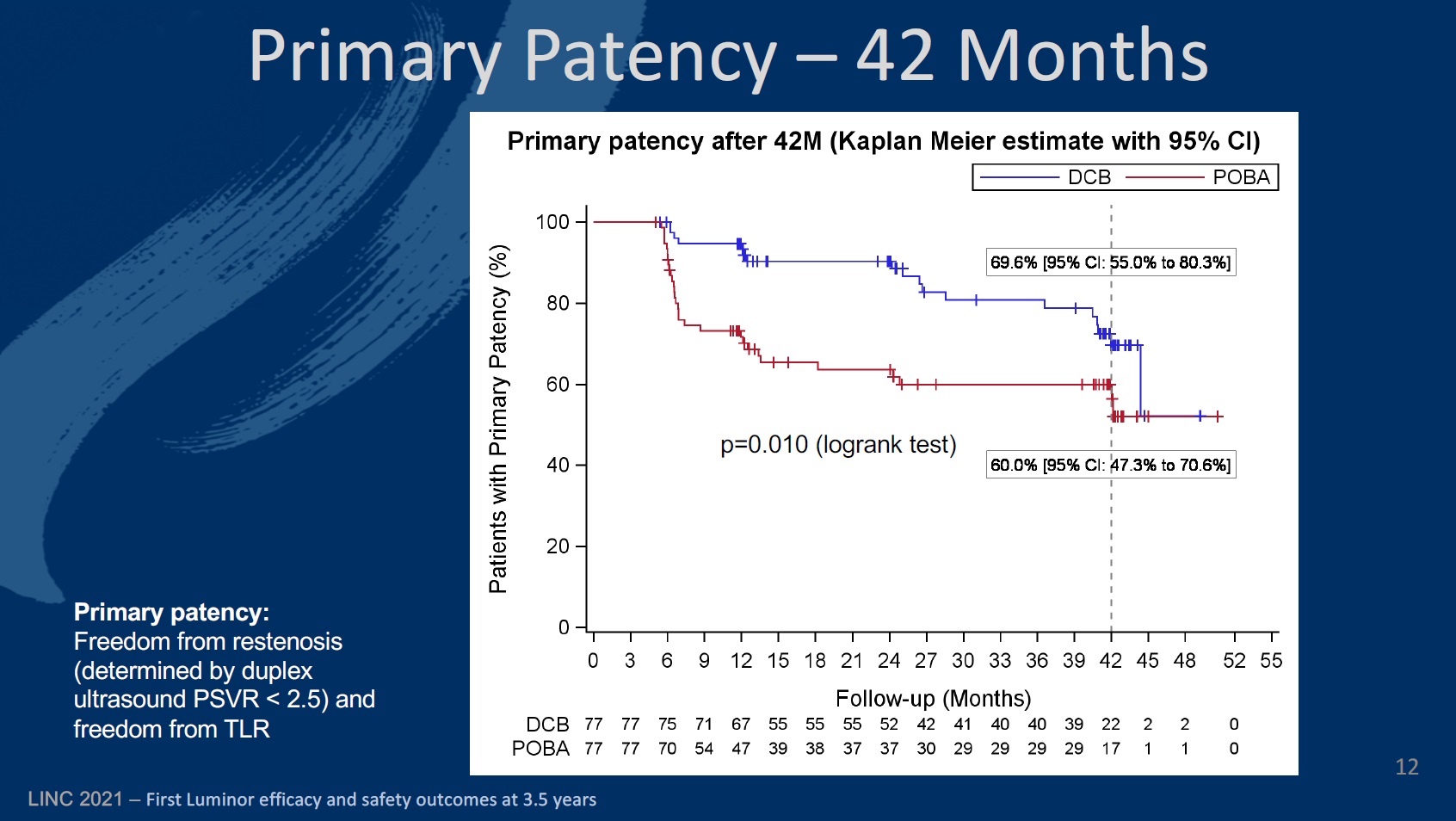
Looking now at the 24-month results, Teichgräber and colleagues reveal that primary patency (the rate of freedom from restenosis) was 90.2% (80.4–95.2) in the DCB arm, compared to 94.7% (86.4–98) at 12 months. The corresponding figures for the PTA cohort were 62.7% (50–73) and 71.3% (59.4–80.3).
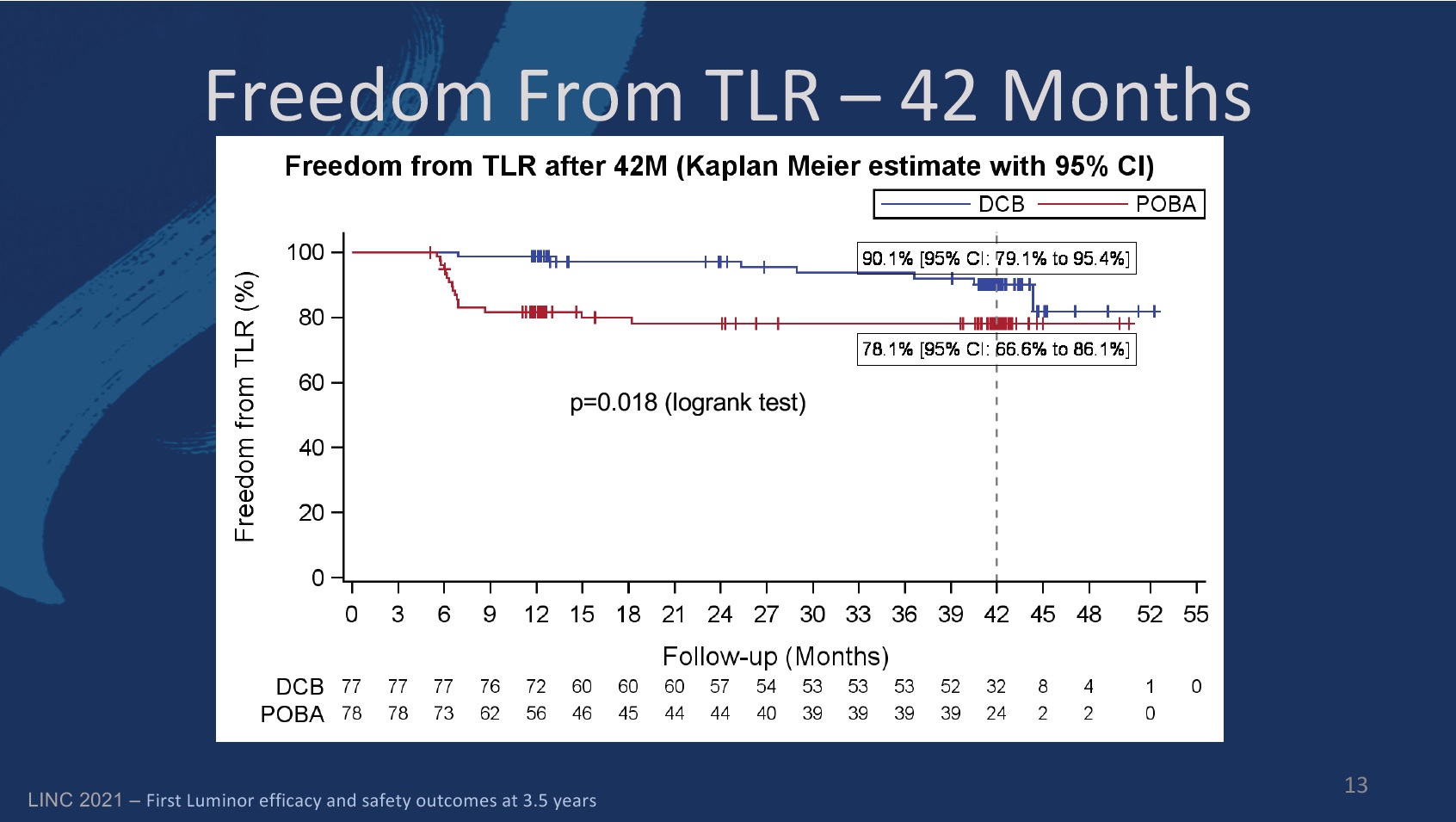
Freedom from TLR in the DCB group was 97.2% (89.1–99.3) at 24 months, compared to 98.7% (91.1–99.8) at 12 months. In the PTA group, the figures were 78% (66.5–86) and 81.6% (71–88.7), respectively.
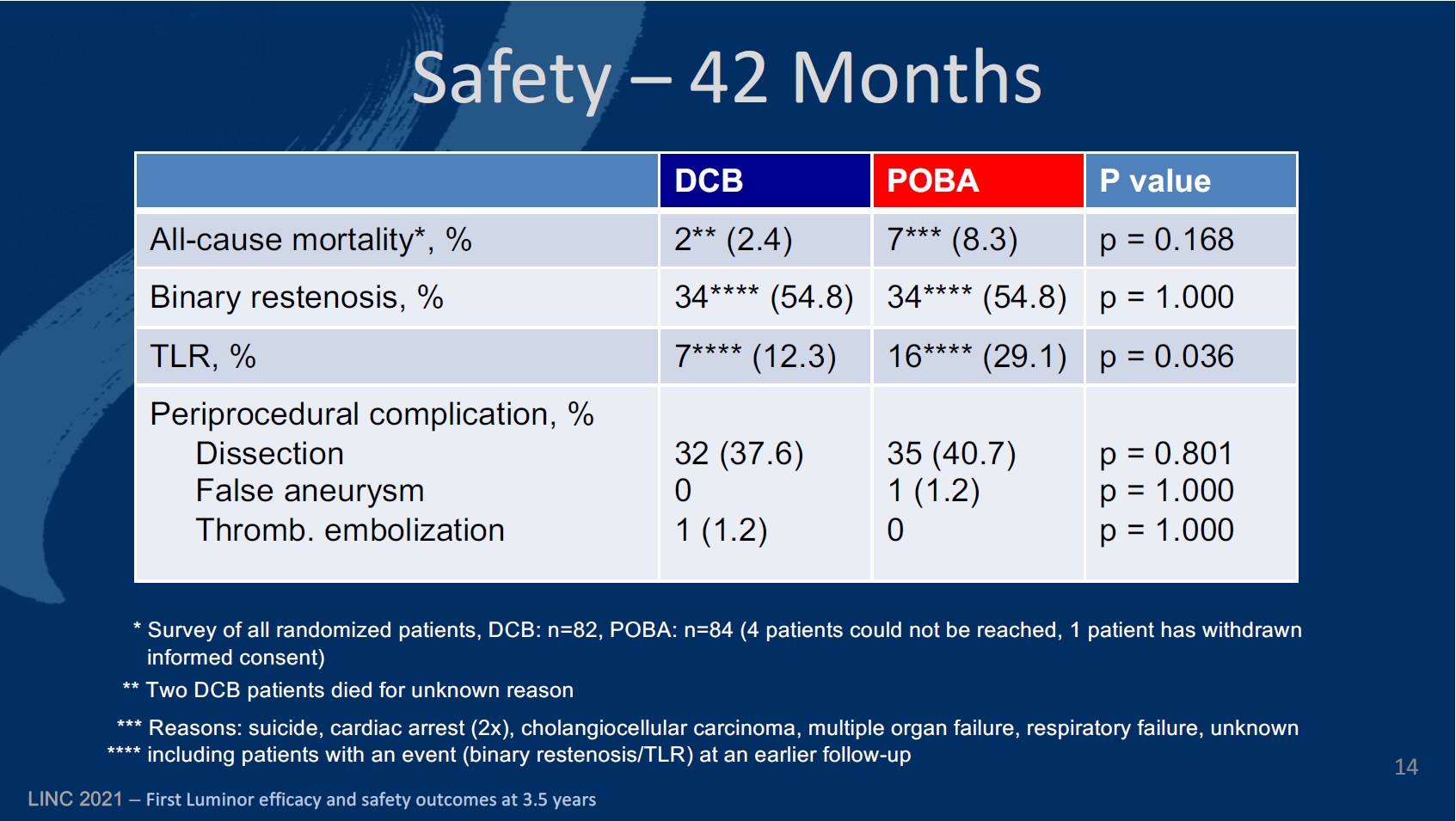
The investigators detail that in both groups patients were of similar age. While the DCB cohort was 68±7.5 years old, on average, the PTA group was 68.1±8.8 (p=0.979). There were slightly fewer male patients in the DCB group (60%) than in the PTA group (69.8%; p=0.239), and also slightly fewer diabetic patients in the DCB group (36.5%), compared to 40.4% in the PTA group (p=0.681). There were fewer current smokers in the PTA group than among DCB patients (40.5% vs. 43%; p=0.856). There were just over double the number of patients with critical limb ischaemia in the DCB group (3.6%) than there were in the PTA group (1.2%; p=0.929).
10.09.2019|72/19|Berlin|
Paclitaxel-beschichtete Produkte wie beschichtete Ballonkatheter (DCB) oder Medikament-freisetzende Stents (DES) bleiben eine primäre Therapieoption für Patienten mit peripherer arterieller Verschlusskrankheit (pAVK) und hohem Restenoserisiko oder Restenosen. Darauf haben Experten des BVMed-Fachbereichs “Periphere Gefäßmedizin” vor dem Hintergrund jüngster Empfehlungen der US-amerikanischen Gesundheitsbehörde (FDA) hingewiesen. In den letzten Monaten hatte eine griechische Metaanalyse von Dr. Katsanos bei Ärzten und Patienten für Verunsicherung gesorgt. Sie enthält aber nach Meinung der BVMed-Experten erhebliche methodische Mängel.
Der BVMed weist darauf hin, dass die FDA in ihrer letzten schriftlichen Mitteilung vom 7. August 2019 Ärzten die Option, pAVK-Patienten mit hohem Restenoserisiko oder Restenosen mittels Paclitaxel-beschichteten Stent- und Ballonkathetern zu therapieren, bestätigt hat. Somit haben pAVK-Patienten weiterhin Zugang zu Paclitaxel-beschichteten Produkten (DCB und DES) als primäre Therapieoption.
Die Wirksamkeit der Produkte wurde im Rahmen von Studien gezeigt. Laut Aussagen der FDA ist die derzeitige Studien- und Erkenntnislage jedoch noch nicht ausreichend, um eine klare Position für oder gegen den Einsatz und Nutzen der Paclitaxel-beschichteten Produkte zu beziehen. Basierend auf der aktuellen Datenlage konnte jedoch kein Zusammenhang zwischen dem Wirkstoff Paclitaxel, der Dosis und der Mortalitätsrate hergestellt werden. Ein Trend pro Paclitaxel sei bei der FDA aber aufgrund jüngster Präsentationen zu erkennen.
Die FDA bestätigt, dass die Vorteile der Behandlung mit DCB und DES die möglichen Risiken überwiegen, wenn bei pAVK-Patienten ein hohes Restenoserisiko und daraus resultierende wiederholte Interventionen diagnostiziert werden. Der BVMed-Fachbereich Periphere Gefäßmedizin unterstützt diese aktuellen Einschätzungen der FDA.
Die weitere Zusammenarbeit von Behörden, Industrie und Ärzten ist wichtig, um die Erkenntnislage zu verfestigen, dass die in der Katsanos-Metanalyse erhöhte Sterbewahrscheinlichkeit in keinem Zusammenhang mit Paclitaxel steht. Die FDA fordert alle Beteiligten auf, an einer belastbaren Langzeitdatenlage zu arbeiten, behandelte Patienten zu beobachten sowie Erkenntnisse und Erfahrungen aus Studien und klinischem Alltag zu berichten.
Zum Hintergrund: In Deutschland leiden über 4,5 Millionen Menschen an einer pAVK. Durch Verengungen oder Verschlüsse der peripheren Arterien werden die Extremitäten dabei nicht mehr ausreichend mit Blut versorgt. Zu über 90 Prozent sind die Beine betroffen. Reicht die konservative Therapie mit Gehtraining nicht aus oder ist die pAVK bereits fortgeschritten, muss die Blutversorgung in den meisten Fällen durch einen operativen Eingriff wieder hergestellt werden. Dies kann durch eine klassische Bypass-Operation erreicht werden. Schonender ist ein minimal-invasiver Eingriff wie das Aufdehnen des verengten Gefäßabschnittes mit Hilfe eines Ballonkatheters (Angioplastie), auch in Kombination mit der Platzierung eines Stents. Je nach der individuellen Situation des Patienten kann der behandelnde Arzt unter verschiedenen Therapieoptionen wie Medikament-freisetzenden oder beschichteten Ballons sowie Medikament-freisetzenden Stents wählen.
Die periphere arterielle Verschlusskrankheit (pAVK) – auch als Schaufensterkrankheit bekannt – sollte nach BVMed-Ansicht so früh wie möglich diagnostiziert und behandelt werden. Bleibt sie längere Zeit unentdeckt, steigt nach Ansicht der Experten das Risiko für schwere Folgen wie Amputation, Schlaganfall und Herzinfarkt.
Referenz-Nr.: 00092/19
Im Dezember 2018 wurde von Katsamos et al. im Journal of American Heart Association eine Meta-Analyse* randomisierter kontrollierter Studien veröffentlicht. Die Analyse konzentrierte sich auf Paclitaxel-beschichtete Ballons und Stents, die in Oberschenkel- und Kniekehlenarterien bei Patientinnen und Patienten mit intermittierender Claudicatio eingesetzt wurden. Die Autoren kamen zu dem Ergebnis, dass Patientinnen / Patienten, bei denen diese Produkte eingesetzt wurden, 2 bis 5 Jahre nach deren Anwendung eine statistisch signifikant erhöhte Sterbewahrscheinlichkeit (Gesamtmortalität) im Vergleich zu Patientinnen / Patienten aufwiesen, die mit unbeschichteten Ballons oder Bare-Metal-Stents behandelt wurden. Da die spezifische Ursache und der Mechanismus der erhöhten Mortalität auch nach Überprüfung der Meta-Analyse von Katsamos et al. momentan noch unbekannt ist, kann nach Auffassung des BfArM derzeit noch keine abschließende Empfehlung zur Verwendung der in Rede stehenden Produkte erfolgen. Hierzu sind weiterführende Datenanalysen notwendig, um insbesondere zusätzliche Langzeitdaten in die Auswertung zu integrieren.
Dennoch zeigen die Daten ein Signal, dass es ab 2 Jahren nach der Anwendung zu einer erhöhten Sterbewahrscheinlichkeit für Patientinnen und Patienten kommen kann, deren periphere arterielle Erkrankung mit Paclitaxel-beschichteten Stents oder Ballons behandelt wurden.
Patientinnen und Patienten sollten sich mit der behandelnden Ärztin / dem behandelnden Arzt in Verbindung setzen, ob ggf. besondere Überwachungsmaßnahmen notwendig werden.
Das BfArM wird weiterhin in engem Austausch mit den europäischen und internationalen Partnerbehörden sowie den medizinischen Fachgesellschaften neue Erkenntnisse zu Risiken prüfen und ggf. über weitergehende Maßnahmenempfehlungen entscheiden und entsprechend informieren.
*Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.
Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D.
J Am Heart Assoc. 2018 Dec 18;7(24):e011245. doi: 10.1161/JAHA.118.011245
Bei etwaigen Rückfragen wenden Sie sich bitte an:
Bundesinstitut für Arzneimittel und Medizinprodukte
Abteilung Medizinprodukte
Kurt-Georg-Kiesinger-Allee 3
53175 Bonn
Telefon (0228) 99 307-3202 (Nichtaktive Medizinprodukte)
Telefax (0228) 99 307-5300
E-Mail: mp-vigilanz@bfarm.de
Stand 13.06.2019
Background
Five years of prospective clinical trials confirm that the paclitaxel drug-coated balloon (DCB) (IN.PACT Admiral, Medtronic, Dublin, Ireland) is safe and effective to treat femoropopliteal artery disease. A recent meta-analysis of heterogeneous trials of paclitaxel-based balloons and stents reported that they are associated with increased mortality and that higher doses are linked to higher mortality from 2 to 5 years.
Objectives
The purpose of this study was to determine if there is a correlation between paclitaxel exposure and mortality by conducting an independent patient-level meta-analysis of 1,980 patients with up to 5-year follow-up.
Methods
Data from 2 single-arm and 2 randomized independently adjudicated prospective studies of a paclitaxel DCB (n = 1,837) and uncoated percutaneous transluminal angioplasty (PTA) (n = 143) were included. Analyses of baseline, procedure, and follow-up data of individual patients were performed to explore correlations of paclitaxel dose with long-term mortality. Survival time by paclitaxel dose tercile was analyzed with adjustment of inverse probability weighting to correct baseline imbalances and study as random effect. A standard cohort was defined to compare DCB- and PTA-treated patients with similar characteristics by applying criteria from pivotal studies (n = 712 DCB, n = 143 PTA).
Results
A survival analysis stratified nominal paclitaxel dose by low, mid, and upper terciles; mean doses were 5,019.0, 10,007.5, and 19,978.2 μg, respectively. Rates of freedom from all-cause mortality between the 3 groups through 5 years were 85.8%, 84.2%, and 88.2%, respectively (p = 0.731). There was no significant difference in all-cause mortality between DCB and PTA through 5 years comparing all patients (unadjusted p = 0.092) or patients with similar characteristics (adjusted p = 0.188).
Conclusions
This independent patient-level meta-analysis demonstrates that this paclitaxel DCB is safe. Within DCB patients, there was no correlation between level of paclitaxel exposure and mortality. (Randomized Trial of IN.PACT Admiral® Drug Coated Balloon vs Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease [INPACT SFA I], NCT01175850; IN.PACT Admiral Drug-Coated Balloon vs. Standard Balloon Angioplasty for the Treatment of Superficial Femoral Artery [SFA] and Proximal Popliteal Artery [PPA] [INPACT SFA II], NCT01566461; MDT-2113 Drug-Eluting Balloon vs. Standard PTA for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery and/or Proximal Popliteal Artery [MDT-2113 SFA], NCT01947478; The IN.PACT SFA Clinical Study for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery and/or Proximal Popliteal Artery Using the IN.PACT Admiral™ Drug-Eluting Balloon in a Chinese Patient Population, NCT02118532; and IN.PACT Global Clinical Study, NCT01609296)
Central Illustration

Paris, May 21st, 2019 – EFFPAC RCT 24-month outstanding results with Luminor DCB have been revealed at EuroPCR 2019.
The 24-month results from the full clinical cohort of the EFFPAC randomized controlled trial (RCT), were presented for the first time at EuroPCR 2019 on May 21st.
Main objective was to assess the effectiveness of Luminor drug coated balloon (DCB) vs. Uncoated balloon catheter (POBA), in the superficial femoral and popliteal arteries. The EFFPAC RCT 24-month outcomes are all demonstrating high statistical significance versus POBA, therefore confirming and even maintaining, the already excellent 12-month outcomes:


To view the complete presentation from Prof. Teichgräber about EFFPAC trial at EuroPCR 2019
« CLICK HERE »
Official article @ iVascular.global:
« CLICK HERE »
Interview from Vascular News with Prof. Teichgräber @EuroPCR 2019:
« CLICK HERE »
NiTiDES is a polymer free stent-eluting the amphilimus formulation (a combination of sirolimus and fatty acid) through the “Abluminal Reservoir Technology” aiming at obtaining the highest efficacy profile for the patient. The polymer-free platform, which is made of nitinol, is integrally covered by the Bio Inducer Surface coating, a second generation pure carbon “ultra thin” layer that drastically increases haemo- and biocompatibility.
Alvimedica NiTiDES – Platform characteristics / Dr. Ralf Langhoff, St. Gertrauden Hospital, Berlin


London, April 25th, 2018 – EFFPAC RCT 12-month outstanding results with Luminor DCB have been revealed at CX Symposium 2018.
The 12-month results from the full clinical cohort of the EFFPAC randomized controlled trial (RCT), were presented for the first time at CX Symposium 2018 at the DCB session on April 24th.
Main objective was to assess the effectiveness of Luminor drug coated balloon (DCB) vs. Uncoated balloon catheter (POBA), in the superficial femoral and popliteal arteries. The EFFPAC RCT 12-month outcomes are all demonstrating high statistical significance versus POBA, therefore confirming and even maintaining, the already excellent 6-month outcomes:
To view the complete presentation from Prof. Teichgräber @ about EFFPAC trial at CharingX 2018
« CLICK HERE »
